| < | ožujak, 2018 | > | ||||
| P | U | S | Č | P | S | N |
| 1 | 2 | 3 | 4 | |||
| 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| 19 | 20 | 21 | 22 | 23 | 24 | 25 |
| 26 | 27 | 28 | 29 | 30 | 31 | |
Srpanj 2020 (1)
Lipanj 2020 (1)
Svibanj 2020 (1)
Travanj 2020 (1)
Ožujak 2020 (1)
Veljača 2020 (1)
Siječanj 2020 (1)
Prosinac 2019 (2)
Srpanj 2019 (1)
Lipanj 2019 (2)
Svibanj 2019 (1)
Travanj 2019 (1)
Veljača 2019 (1)
Prosinac 2018 (2)
Studeni 2018 (1)
Rujan 2018 (4)
Srpanj 2018 (1)
Ožujak 2018 (3)
Veljača 2018 (1)
Prosinac 2017 (3)
Studeni 2017 (2)
Links
Project forum
Website of the project
Općenito o projektu
Prvi meeting u Poljskoj
Agencija za mobilnost i programe EU
You Tube Channel of the project
Website of the project
Općenito o projektu
Prvi meeting u Poljskoj
Agencija za mobilnost i programe EU
You Tube Channel of the project
VIDEOS FROM THE MEETING IN CROATIA
30.03.2018., petak
Hello, it's Earth!
Activity for March - HELLO, IT'S EARTH!
What did young biologists do in Croatian Natural History Museum in Zagreb?
We visited Croatian Natural History Museum in Zagreb to participate in a workshop called „CSI CNHM– Chicken coop crime“. The workshop included a detailed survey of: a structure and position of DNA in a cell, DNA role in heredity, basic analysis methods (DNA laboratory) and implementation of DNA in forensic science (DNA detective).
Our students recorded a movie "behind the scenes" (we've participated in the presence of Croatian Radiotelevision in children's show „Luka and friends“.)
Enjoy in the movie
Hello, it's EARTH!
28.03.2018., srijeda
MEETING OF GLOBE ELEMENTARY SCHOOLS IN SLATINA
Students of Josip Kozarac Elementary School and Eugen Kumičić Elementary School, along with their teachers- mentors, achieved cooperation within the GLOBE program. Both schools have been actively implementing the GLOBE program for a long period of time which is necessary to fulfill certain conditions in school and purchase necessary equipment. Measurements and observations made in our environment include area of the atmosphere, water, soil and vegetation (plant cover). Both schools put the gathered results into a joint database on the GLOBE server. We are a part of a big GLOBE family which consists of over 20 000 schools in over 100 countries all over the world on all continents, including the Antarctica.
(Croatia was among the first countries to enter the GLOBE program and is currently one of the hundred countries included in the program which, according to percentage, ranks us among the first countries in the world.)
At this meeting, our school got a chance to see the work of the Eugen Kumičić Elementary School through presentations and workshops which made by students with the help of their teachers-mentors. There was a demonstration of water analysis taken out of Slatina's Javorica Lake where the students measured the amount of oxygen, presence of nitrites and pH values by using the IabDisc.
17.03.2018., subota
How much Physics in Chemistry?
Dana 28.veljače, održana je radionica iz Fizike i Kemije na temu How much Physics in Chemistry? Radionica je bila namijenjena učenicima šestih razreda. Učenici su pomoću pribora koji se koriste i u Kemiji i u Fizici izvodili sljedeće pokuse:
a) određivanje obujma nepravilnog tijela
Učenicima je ponuđeno nekoliko tijela nepravilnog oblika (kamen, uteg i komad plastelina). Učenici su određivali obujam zadanih tijela koristeći se principom Arhimedovog zakona. Kao mjerni instrument ponuđena im je menzura s vodom. Učenici su samostalno, metodom pretpostavki; došli do zaključka kako izmjeriti obujam nepravilnog tijela uranjanjem u vodu. Kasnije su mijenjanjem oblika plastelinu, provjerili utječe li promjena oblika tijela na promjenu obujma.
b) toranj gustoće
Učenici su od kuće donijeli nekoliko tekućina različitih boja. Cilj ovoga pokusa je bio da pokušaju uliti što više različitih tekućina u menzuru bez da se one pomiješaju. Koristili smo: vodu, ulje, sredstvo za pranje suđa, antifriz i šampon. Ulijevajući razne tekućine u menzuru one ostaju u slojevima zbog različitosti svojih gustoća.
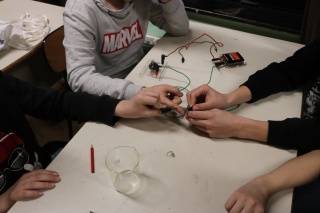
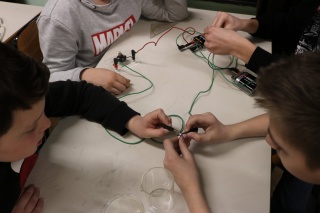
c) provodljivost krutih tvari i tekućina
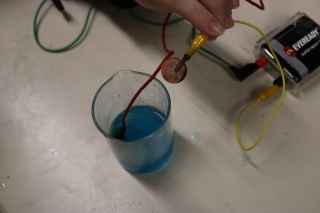
Učenici su zatvarali strujni krug s predmetima iz svojih pernica. Zaključili su da metali dobro provode struju. Zatim su ispitivali provodljivost različitih tekućina. Primijetili su da voda bolje provodi struju ako se posoli, također su uočili se na jedan od vodiča skupljaju mjehurići. Kada su ispitivali provodljivost vodene otopine modre galice na jedan uronjeni vodič stavili su novčić. Novčić je postao bakren razlaganjem kemijskog spoja vodene otopine modre galice pomoću struje na elemente.
Pokuse osmislile nastavnica fizike Martina Bukvić i nastavnica kemija Ivana Kotarski Milek
On 28th of February there was a workshop for 6th grade students with the topic ‘How much Physics in Chemistry?’ Students used equipment that is used in both physics and chemistry to conduct the following experiments:
a) Detection of the volume of an irregular body
Students were offered a few irregularly shaped bodies (a rock, a weight, a piece of plasticine) and they were supposed to detect the given bodies volume by using the principle of the Archimedes law. They were also given a graduated cylinder with water as a measuring instrument. Students came to their conclusions independently by using the method of assumption and they realized that they should put the object in water and then measure its volume. Later on, they changed the shape of the plasticine and checked if it influences the volume of the irregular body.
b) Tower of density
Students brought a few differently colored liquids from home and the goal of this experiment was to put in as many different liquids as possible in the graduated cylinder without them mixing. We used: water, oil, dish soap, shampoo and antifreeze liquid. Pouring in different types of liquids makes them stay in layers because each of them has different density.
c) Conductivity of solids and liquids
Students closed the electric circuit with various objects out of their pencil cases. They realized that metals conduct electricity the best. Then they examined the conductivity of different liquids. The students noticed that water will conduct electricity better if they put a little salt in it and they also noticed that bubbles appear with one of the conductors. When they examined the conductivity water solution of cupric sulfate anhydrous on one of the immersed conductors they put a coin on it. The coin became copper because of the decomposition into elements of the chemical compound of water solution of cupric sulfate anhydrous by using electricity.
The experiments were created by Physics teacher Martina Bukvić and Chemistry teacher Ivana Kotarski Milek